However, it took another 120 years until NASA demonstrated its use to provide electricity and water for some early space flights. Today the fuel cell is the primary source of electricity on the space shuttle. As a result of these successes, industry slowly began to appreciate the commercial value of fuel cells. In addition to stationary power generation applications, there is now a strong push to develop fuel cells for automotive use.
Even though fuel cells provide high performance characterisitics, reliability, durability, and environmental benefits, a very high investment cost is still the major barrier against large-scale deployments.
Basic Principles
The fuel cell works by processing a hydrogen-rich fuel – usually natural gas or methanol – into hydrogen, which, when combined with oxygen, produces electricity and water. This is the reverse electrolysis process. Rather than burning the fuel, however, the fuel cell converts the fuel to electricity using a highly efficient electrochemical process. A fuel cell has few moving parts, and produces very little waste heat or gas.A fuel cell power plant is basically made up of three subsystems or sections. In the fuel-processing section, the natural gas or other hydrocarbon fuel is converted to a hydrogen-rich fuel. This is normally accomplished through what is called a steam catalytic reforming process. The fuel is then fed to the power section, where it reacts with oxygen from the air in a large number of individual fuel cells to produce direct current (DC) electricity, and by-product heat in the form of usable steam or hot water.
For a power plant, the number of fuel cells can vary from several hundred (for a 40-kW plant) to several thousand (for a multi-megawatt plant). In the final, or third stage, the DC electricity is converted in the power conditioning subsystem to electric utility-grade alternating current (AC).
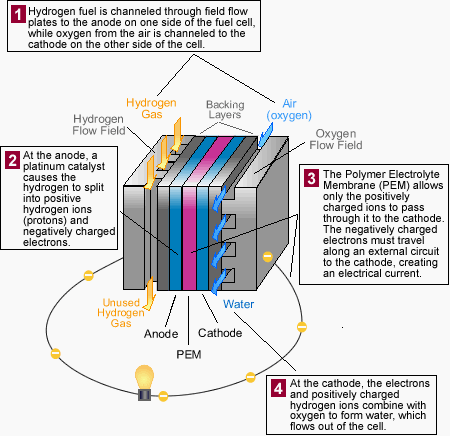
Fuel cell - How it works
In the reduction half-reaction at the cathode, oxygen supplied from air combines with the hydrogen ions and electrons to form water and excess heat.
Thus, the final products of the overall reaction are electricity, water, and excess heat.
Types of Fuel Cells
The electrolyte defines the key properties, particularly the operating temperature, of the fuel cell. Consequently, fuel cells are classified based on the types of electrolyte used as described below.- Polymer Electrolyte Membrane (PEM)
- Alkaline Fuel Cell (AFC)
- Phosphoric Acid Fuel Cell (PAFC)
- Molten Carbonate Fuel Cell (MCFC)
- Solid Oxide Fuel Cell (SOFC)
TABLE 1 – Comparison of Five Fuel Cell Technologies
| Type | Electrolyte | Temperature C | Applications | Advantages |
| Polymer Electrolyte Membrane (PEM) | Solid organic polymer poly-perflourosulfonic acid | 60–100 | Electric utility, transportation, portable power | Solid electrolyte reduces corrosion, low temperature, quick start-up |
| Alkaline (AFC) | Aqueous solution of potassium hydroxide soaked in a matrix | 90–100 | Military, space | Cathode reaction faster in alkaline electrolyte; therefore high performance |
| Phosphoric Acid (PAFC) | Liquid phosphoric acid soaked in a matrix | 175–200 | Electric utility, transportation, and heat | Up to 85% efficiency in cogeneration of electricity |
| Molten Carbonate (MCFC) | Liquid solution of lithium, sodium, and/or potassium carbonates soaked in a matrix | 600–1000 | Electric utility | Higher efficiency, fuel flexibility, inexpensive catalysts |
| Solid Oxide (SOFC) | Solid zirconium oxide to which a small amount of yttria is added | 600–1000 | Electric utility | Higher efficiency, fuel flexibility, inexpensive catalysts. Solid electrolyte advantages like PEM |



0 comments:
Post a Comment